| Background | Diffraction |
Crystal Quality | Facilities |
| Home |
Proteins |
| Neural Networks |
Diffracting Proteins
The procedure for determining the three-dimensional structure of a protein is called Protein Crystallography. The first step is to grow the protein into a crystal. This is accomplished by isolating the protein with a centrifuge and then purifying it. Using the vapor pressure method, crystals are formed that contain the protein at every lattice point. These procedure is extremely problematic; protein crystals are difficult to grow,difficult to transport and they deteriorate rapidly. Several hundred must be grown and screened in order to have one or two usable crystals.

Once a usable crystal is found, it must be diffracted. When energetic X-rays pass through protein crystals, they are deflected by the protein molecule's electron clouds. Because crystals have a repeating, symmetric formation, the deflected X-rays cause constructive interference with one another. These X-rays are absorbed as a pattern of dots called a diffraction pattern by a photon detector. If a protein is diffracted from all angles, analysis of the diffraction patterns will provide a template of the electron densities within the protein.

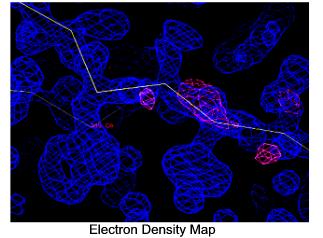
The final step in determining the three-dimensional structure of a protein crystal is to "fit" the electron density map with amino acids. Since proteins are comprised of chains of amino acids, the electron densities of the amino acids are fitted into the electron density map of the protein by a computer. The process is like fitting the pieces of a puzzle together. Using small wavelength X-rays creates very fine, high resolution electron maps which allow the amino acids be accurately placed.